atom
What is an atom?
An atom is a particle of matter that uniquely defines a chemical element. An atom consists of a central nucleus that is surrounded by one or more negatively charged electrons. The nucleus is positively charged and contains one or more relatively heavy particles known as protons and neutrons.
Atoms are the basic building blocks of matter. Anything that takes up space and anything with mass is made up of atoms.
What are protons and neutrons?
Protons and neutrons are subatomic particles that make up the center of the atom, or its atomic nucleus.
- A proton is positively charged. The number of protons in the nucleus of an atom is the atomic number for the chemical element. Different elements' atomic numbers are found in the Periodic Table of Elements. For example, sodium has 11 protons, and its atomic number is 11. A proton has a rest mass, denoted mp, of approximately 1.673 x 10-27 kilogram (kg).
- A neutron is electrically neutral and has a rest mass, denoted mn, of approximately 1.675 x 10-27.
The mass of a proton or neutron increases when the particle attains extreme speed, for example in a cyclotron or linear accelerator.
The structure of an atom
The total mass of an atom, including the protons, neutrons and electrons, is the atomic mass or atomic weight. The atomic mass or weight is measured in atomic mass units.
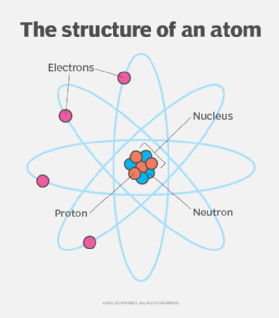
Electrons contribute only a tiny part to the mass of the atomic structure, however, they play an important role in the chemical reactions that create molecules. For most purposes, the atomic weight can be thought of as the number of protons plus the number of neutrons. Because the number of neutrons in an atom can vary, there can be several different atomic weights for most elements.
Protons and electrons have equal and opposite charges. Protons have a positive charge and electrons a negative charge. Normally, atoms have equal numbers of protons and electrons, giving them a neutral charge.
An ion is an atom with a different number of electrons than protons and is electrically charged. An ion with extra electrons has a negative charge and is called an anion and an ion deficient in electrons has a positive charge and is called a cation.
Atoms having the same number of protons but different numbers of neutrons represent the same element and are known as isotopes of that element. An isotope for an element is specified by the sum of the number of protons and neutrons. For example, the following are two isotopes of the carbon atom:
- Carbon 12 is the most common, non-radioactive isotope of carbon.
- Carbon 14 is a less common, radioactive carbon isotope.
The only neutral atom with no neutrons is the hydrogen atom. It has one electron and one proton.
History of the atom
According to CERN, which is the European Council for Nuclear Research, atoms were created 13.7 billion years ago in the first few minutes after the Big Bang. The new universe cooled and expanded, creating the conditions for electrons and quarks -- the smaller particles that make up protons and neutrons -- to form. Millionths of a second later, quarks aggregated to form protons and neutrons, which combined to form the nuclei of atoms.
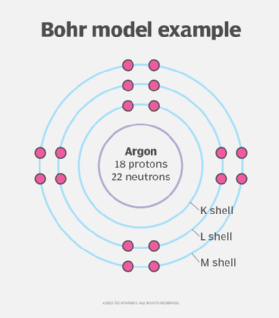
The physicist Ernest Rutherford developed an early model of the atom in 1912. He was the first to suggest that atoms are like miniature solar systems, except that instead of gravity acting as the attractive force, opposing electrical charges serve that function. In the Rutherford atom of atomic theory, electrons orbit the nucleus in circular paths.
Another physicist, Niels Bohr, revised Rutherford's atomic model in 1913. The Bohr atom included negatively charged electrons orbiting the nucleus at specific median distances. These distances are represented by spheres, called shells, surrounding the nucleus. Electrons can move from shell to shell. When an electron absorbs enough energy, it moves to a larger, or higher, shell. When it loses a certain amount of energy, it falls to a smaller, lower shell.
The Bohr radius constant is based on Bohr's model of the atom.
Atomic power
A strong nuclear force holds together the protons and neutrons in the nucleus of an atom. That force overcomes the repulsive force between the positively charged particles. Strong nuclear force -- sometimes referred to as strong force or strong interaction -- only works at very close distances. Strong force is the strongest of the four fundamental forces in nature; the other three are gravitational, electromagnetic and weak nuclear forces.
When the bond between particles in the nucleus is broken, a large amount of energy is released. The process of breaking these bonds is known as nuclear fission. Nuclear power plants use fission to split uranium atoms and generate electricity. Uranium is used for fission because its atoms split relatively easily.
Nuclear power is considered a clean energy source because fission does not emit greenhouse gases. It is a possible energy source for IT data centers looking to reduce their carbon footprint.
Learn about the role atomic physics plays in quantum computing and why you should pay attention to these developments.
